VIVO Pathophysiology
The Gastrointestinal Barrier
The gastrointestinal mucosa forms a barrier between the body and a lumenal environment which not only contains nutrients, but is laden with potentially hostile microorganisms and toxins. The challenge is to allow efficient transport of nutrients across the epithelium while rigorously excluding passage of harmful molecules and organisms into the animal. The exclusionary properties of the gastric and intestinal mucosa are referred to as the "gastrointestinal barrier".
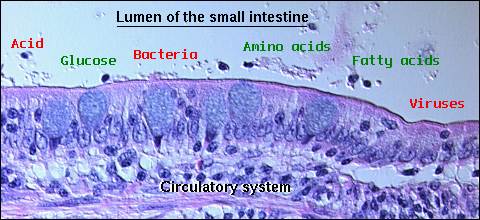
It is clear that a number of primary gastrointestinal diseases lead to disruption of the mucosal barrier, allowing escalation to systemic disease. It is equally clear that many systemic disease processes result in damage to the gastrointestinal barrier, thereby adding further insult to an already compromised system. Understanding the nature of the barrier can assist in predicting such events and aid in prophylactic or active therapies.
The gastrointestinal barrier is often discussed as having two components:
- The intrinsic barrier is composed of the epithelial cells lining the digestive tube and the tight junctions that tie them together.
- The extrinsic barrier consists of secretions and other influences that are not physically part of the epithelium, but which affect the epithelial cells and maintain their barrier function.
The Intrinsic Gastrointestinal Barrier
The alimentary canal is lined by sheets of epithelial cells that form the defining structure of the mucosa. With few exceptions, epithelial cells in the stomach and intestines are circumferentially tied to one another by tight junctions, which seal the paracellular spaces and thereby establish the basic gastrointestinal barrier. Throughout the digestive tube, maintenance of an intact epithelium is thus critical to the integrity of the barrier. In general, toxins and microorganisms that are able to breach the single layer of epithelial cells have unimpeded access to the the systemic circulation.
As might be anticipated, there is diversity among different types of epithelial cells in specific barrier functions. For example, the apical plasma membranes of gastric parietal and chief cells have atypically low permeability to protons, which aids in preventing damage due to back diffusion of acid into the cells. Small intestinal epithelial cells lack this specialized ability and thus are much more susceptible to acid-induced damage.
Tight junctions encircling gastrointestinal epithelial cells are a critical component of the intrinsic barrier. These structures used to be viewed as passive structures akin to welds, but recent studies indicate that they are much more dynamic than previously thought, and their permeability may be regulated by a number of factors that affect the epithelial cells.
The gastrointestinal epithelium is populated by a variety of functionally-mature cells derived from proliferation of stem cells. Most of the mature epithelial cells, including mucous cells in the stomach and absorptive cells in the small intestine, show rapid turnover rates, and die within only a few days after their formation. Maintenance of epithelial integrity thus requires a precise balance between cell proliferation and cell death.
Stem cells that support continual replenishment of gastrointestinal epithelium reside in the middle of the gastric pits and within the crypts of the small and large intestine. Epithelial cell dynamics of the small intestine have been particularly well studied. These stem cells proliferate continually to supply cells that then differentiate into absorptive enterocytes, mucus-secreting goblet cells, enteroendocrine cells and Paneth cells. Except for Paneth cells, which remain in the crypts, the other cells differentiate into their mature forms as they migrate up from the crypts to replace cells extruded from the tips of the villi. This migration takes approximately 3 to 6 days.
The Extrinsic Gastrointestinal Barrier
Mucus and Bicarbonate
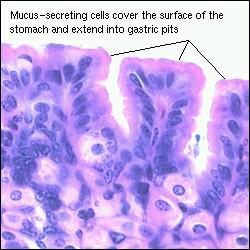
The entire gastrointestinal epithelium is coated with mucus, which is synthesized by cells that form part of the epithelium. Mucus serves an important role in mitigating shear stresses on the epithelium and contributes to barrier function in several ways. The abundant carbohydrates on mucin molecules bind to bacteria, which aids in preventing epithelial colonization and, by causing aggregation, accelerates clearance. Diffusion of hydrophilic molecules is considerably lower in mucus than in aqueous solution, which is thought to retard diffusion of a variety of damaging chemicals, including gastric acid, to the epithelial surface.
In addition to being coated with a mucus layer, gastric and duodenal epithelial cells secrete bicarbonate ion on their apical faces. This serves to maintain a neutral pH along the epithelial plasma membrane, even though highly acidic conditions exist in the lumen.
Hormones and Cytokines
Normal proliferation of gastric and intestinal epithelial cells, as well as proliferation in response to such injury as ulceration, is known to be affected by a large number of endocrine and paracrine factors. Several of the enteric hormones are known to enhance rates of proliferation. Different forms of injury to the epithelium can lead to either enhanced or suppressed rates of cell proliferation. For example, it has been demonstrated that resection of a portion of the canine small intestine is followed by epithelial cell hyperplasia and increased villous length in animals fed orally. Animals fed parenterally failed to show the same compensatory hyperplasia, indicating that, among other factors, local nutrients play an important role in cell dynamics.

Prostaglandins, particularly prostaglandin E2 and prostacyclin, have long been known to have "cytoprotective" effects on the gastrointestinal epithelium. A common clinical correlate in many mammals is that use of aspirin and other non-steroidal antiinflammatory drugs (NSAIDs) which inhibit prostaglandin synthesis is commonly associated with gastric erosions and ulcers. Dogs are particularly sensitive to this side effect. Prostaglandins are synthesized within the mucosa from arachidonic acid, through the action of cyclooxygenases. Their cytoprotective effect appears to result from a complex ability to stimulate mucosal mucus and bicarbonate secretion, to increase mucosal blood flow and, particularly in the stomach, to limit back diffusion of acid into the epithelium. Considerable effort is underway to develop NSAIDs that fail to inhibit mucosal prostaglandin synthesis.
Two peptides that have received attention for their potential role in barrier maintenance are epidermal growth factor (EGF) and transforming growth factor-alpha (TGF-alpha). EGF is secreted in saliva and from duodenal glands, while TGF-alpha is produced by gastric epithelial cells. Both peptides bind to a common receptor and stimulate epithelial cell proliferation. In the stomach, they also enhance mucus secretion and inhibit acid production. Other cytokines such as fibroblast growth factor and hepatocyte growth factor have been shown to enhance healing of gastrointestinal ulcers in experimental models.
Trefoil proteins are a family of small peptides that are are secreted abundantly by goblet cells in the gastric and intestinal mucosa, and coat the apical face of the epithelial cells. Their distinctive molecular structure appears to render them resistant to proteolytic destruction. A number of studies have demonstrated that trefoil peptides play an important role in mucosal integrity, repair of lesions, and in limiting epithelial cell proliferation. They have been shown to protect the epithelium from a broad range of toxic chemicals and drugs. Trefoil proteins also appear to be a central player in the restitution phase of epithelial damage repair, where epithelial cells flatten and migrate from the wound edge to cover denuded areas. Mice with targeted deletions in trefoil genes showed exaggerated responses to mild chemical injury and delayed mucosal healing.
Another molecule that plays a crucial role in mucosal integrity and barrier function is nitric oxide (NO). Paradoxically, NO also contributes to mucosal injury in a number of digestive diseases. This molecule is synthesized from arginine through the action of one of three isoforms of nitric oxide synthease (NOS). Much of the research in this area has focused on understanding the effects of applying NO donors such as glyceryltrinitrate or NOS inhibitors. In several models, NO donors significantly reduced the severity of mucosal injury induced by toxic chemicals (e.g. ethanol) or associated with ischemia and reperfusion. Similarly, healing of gastric ulcers in rats has been accelerated by application of NO donors. Another intriguing observation is that co-administration of NO donors and NSAIDs results in anti-inflammatory properties comparable to NSAIDs alone, but with less damage to the gastrointestinal mucosa. NOS inhibitors are under investigation for treatment of situations in which NO is overproduced and contributes to mucosal injury.
Antibiotic Peptides and Antibodies
An important part of barrier function is to prevent transit of bacteria from the lumen through the epithelium. Paneth cells are epithelial granulocytes located in small intestinal crypts of many mammals. They synthesize and secrete several antimicrobial peptides, chief among them isoforms of alpha-defensins known also as cryptdins ("crypt defensin"). These peptides have antimicrobial activity against of number of potential pathogens, including several genera of bacteria, some yeasts and Giardia trophozoites. Their mechanism of action is likely similar to neutrophilic alpha-defensins, which permeabilize target cell membranes.
In addition to non-specific antimicrobial molecules, barrier function is supported by the gastrointestinal immune system. One facet of this defense systems is that much of the epithelium is bathed in secretory immunoglobulin A. This class of antibody is secreted from subepithelial plasma cells and transcytosed across the epithelium into the lumen. Lumenal IgA provides an antigenic barrier by binding bacteria and other antigens. This barrier function is specific for particular antigens and requires previous exposure for development of the response.
Disruption of Barrier Function
Despite its robust and multi-faceted nature, the gastrointestinal barrier can be breached. Local infections by bacteria and virus, exposure to toxins or physical insults, and a variety of systemic diseases lead to its disruption. Such problems can be mild and readily repaired, or massive and fatal.
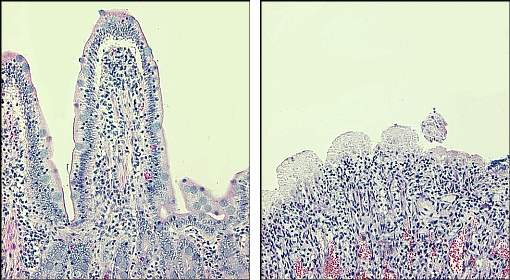
The micrographs below depict severe disruption of the barrier. On the left is mucosa from a normal canine small intestine, with large villi covered by intact epithelium extended into the lumen. The image on the right (same magnification) shows small intestinal mucosa from a dog that died of Salmonella enteritis - note the totally denuded epithelium and destruction of villi.
Ischemia and Reperfusion Injury
Damage to the gastrointestinal barrier due to ischemia and reperfusion injury is a common and serious condition. Ischemia occurs when blood flow is insufficient to deliver an amount of oxygen and nutrients necessary for maintenance of cell integrity. Reperfusion injury occurs when blood flow is restored to ischemic tissue.
Gastrointestinal ischemia results from two fundamental types of disorders, both of which can compromise the epithelial barrier:
- Non-occlusive ischemia results from systemic conditions such as circulatory shock, sepsis or cardiac insufficiency.
- Occlusive ischemia refers to conditions that directly disrupt gastrointestinal blood flow, such as strangulation, volvulus or thromboembolism.
Reperfusion injury to the gastrointestinal wall, especially the mucosa, is thought to be due primarily to generation of reactive oxygen species, including superoxide, hydrogen peroxide and hydroxyl radicals. These oxidants are generated within the mucosa and also in the numerous local leukocytes activated during the course of ischemia.
Oxygen-derived free radicals generated during reperfusion initiate a series of events that causes mucosal damage and disruption of the barrier. They directly damage cell membranes by forming lipid peroxides, which also leads to production of a number of inflammatory mediators derived from phospholipids (e.g. platelet-activiating factor and leukotrienes). These proinflammatory agents function as chemoattractants for neutrophils, which migrate into the mucosa, release their own reactive oxygen metabolites and cause further damage to the intrinsic epithelial barrier. An initially minor effect from ischemia is thus amplified into very significant damage to barrier function. Additionally, the inflammatory mediators generated in the gastrointestinal tract can harm distant tissues, leading to systemic disease.
The observed effects of ischemia-reperfusion injury range from increased vascular permeability and consequent subepithelial edema, to massive loss of epithelial cells and villi. Even relatively mild damage to the epithelium disrupts barrier function and can lead to translocation of bacteria and toxins from the lumen to the systemic circulation. A number of treatments are under development and testing to prevent this cascade of damage, including application of antioxidants such as superoxide dismutase and use of drugs such as platelet-activating factor antagonists to block the effect of inflammatory mediators.
Neutrophils and Mucosal Injury
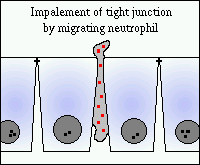
Diverse insults to the intestinal mucosa, including infectious processes, ischemia and damaging chemicals, promote infiltration of neutrophils. This common endpoint results because many types of injuries lead to local production of neutrophil chemoattractants such as leukotrienes, interleukins and activated complement components. In response to chemoattractants, neutrophils migrate out of capillaries, infiltrate the subepithelial mucosa and often transmigrate through the gastric or intestinal epithelium. In crossing the epithelium, neutrophils must break junctional complexes between epithelial cells. This "impalement" through tight junctions necessarily causes transient increases in permiability. When the insult is minor, the junctions reseal quickly, but transmigration of large numbers of neutrophils induces significant damage to barrier function.
Effects of Stress
Stress comes in a myriad of forms and is an integral part of all illness and trauma. The stress response involves modulation of literally dozens of hormones and cytokines, as well as significant effects on neurotransmission. However, the foremost effect of stress on the gastrointestinal tract is to decrease mucosal blood flow and thereby compromise the integrity of the mucosal barrier. Among other things, reduced mucosal blood flow suppresses production of mucus and limits the ability to remove back diffusing protons. As a consequence, significant stress is almost always associated with mucosal erosions, particularly in the stomach. A majority of these lesions are subclinical, but gastrointestinal hemorrhage and sepsis are not infrequent consequences.
Diabetes and obesity are well known risk factors for dysfunction of the GI barrier and the prolonged hyperglycemia associated with these disorders may be the primary mechanism for such disruption.
Restitution and Healing After Injury
The critical first task following disruption of the gastrointestinal epithelium is to cover the denuded area and re-establish the intrinsic barrier. This rapid restoration of epithelium is accomplished by a process called restitution - epithelial cells adjacent to the defect flatten and migrate over the exposed basement membrane. In the small intestine, this process is aided by a rapid contraction and shortening of the affected villi, which reduces the area of basement membrane that must be covered.
Restitution provides a rapid mechanism for covering a defect in the barrier and does not involve proliferation of epithelial cells. It results in an area that, while protected, is not physiologically functional. Healing requires that the epithelial cells on the margins of the defect proliferate, differentiate and migrate into the damaged area to restore the normal cellular architecture and function.
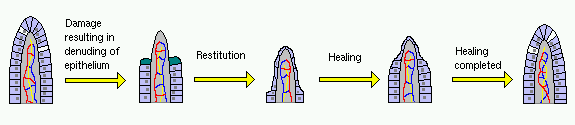
Restitution has been shown to be stimulated by a number of mostly paracrine regulators. Local prostaglandins and trefoil proteins are clearly involved in this process, and suppression of their production significantly delays restitution. Another group of molecules involved in restitution is the polyamines such as spermine, spermidine and putrescine. These molecules are present in many diets and also synthesized by the gastrointestinal mucosa. Enteral administration of polyamines has been shown in experimental models to accelerate restitution and healing of mucosal lesions.
References and Reviews
- Allaire JM, Crowley SM, Law HT, etc. The intestinal epithelium: Central coordinator of mucosal immunity. Trends Immunol 2018, DOI: https://doi.org/10.1016/j.it.2018.04.002
- Blikslager AT, Roberts MC: Mechanisms of intestinal mucosal repair. J Am Vet Med Assoc 211:1437-1441, 1997.
- Dieckgraefe BK, Stenson WF, Alpers DH: Gastrointestinal epithelial response to injury. Curr Opin Gastroenterol 12:109-114, 1996.
- Engle E, Guth PH, Nishizaki Y, Kaunitz JD: Barrier function of gastric mucus gel. Am J Physiol 269:G994-999, 1995.
- Filep J, Herman F, Braquet P, Mozes T: Increased levels of platelet-activiating factor in blood following intestinal ischemia in the dog. Biochem Biophys Res Commun 158:353-355, 1989.
- Gayle JM, Blikslager AT, Jones SL: Role of neutrophils in intestinal mucosal injury. J Am Vet Med Assoc 217:498-500, 2000.
- Kubes P, Hunter J, Granger ND: Ischemia/reperfusion-induced feline intestinal dysfunction: importance of granulocyte recruitment. Gastroenterology 103:807-812, 1992.
- Lichtenberger LM: The hydrophobic barrier properties of gastrointestinal mucus. Ann Review Physio 57:565-583, 1995.
- Mashimo H, Wu D, Podolsky DK, Fishman M: Impaired defense of intestinal mucosa in mice lacking intestinal trefoil factor. Science 274:262-264, 1996.
- Murphy MS: Growth factors and the gastrointestinal tract. Nutrition 14:771-774, 1998.
- Muscara MN, Wallace JL: Nitric oxide V. Therapeutic potential of nitric oxide donors and inhibitors. Am J Physiol 276:G1313-1316, 1999.
- Thaiss CA, Levy M, Grosheva I, etc. Hyperglycemia derives intestinal barrier dysfunction and risk for enteric infection. Science 359:1376-82, 2018.
- Thompson JS: The intestinal response to critical illness. Am J Gastroenterology 90:190-200, 1995.
- Wallace JL, Miller MJ: Nitric oxide in mucosal defense: a little goes a long way. Gastroenterology 119:515-520, 2000.
Updated May 2018. Send comments to Richard.Bowen@colostate.edu
A Czech translation of this page was created by Maxwell Edward and is available at Czech translation
A Dutch translation of this page was created by Umer Anees and is available at Dutch translation
A French translation of this page was created by Erin Melissa and is available at French translation
A Greek translation of this page was created by Vouchers Tree and is available at Greek translation
A Hindi translation of this page was created by Nikol Barton and is available at Hindi translation
A Portuguese translation of this page was created by Hamza Ahmed and is available at Portuguese translation
A Punjabi translation of this page was created by Bydiscountcodes Team and is available at Punjabi translation
A Romanian translation of this page was created by Sarah Richards of Essay Writing Service and is available at Romanian translation
A Spanish translation of this page was created by Paul Diaz and is available at Spanish translation
A Ukrainian translation of this page was created by Sergey Cosbuk of PickWriters and is available at Ukranian translation
An Urdu translation of this page was created by Ahsan Soomro and is available at Urdu translation
