VIVO Pathophysiology
Other Topics
Free Radicals and Reactive Oxygen
A radical (often, but unnecessarily called a free radical) is an atom or group of atoms that have one or more unpaired electrons. Radicals can have positive, negative or neutral charge. They are formed as necessary intermediates in a variety of normal biochemical reactions, but when generated in excess or not appropriately controlled, radicals can wreak havoc on a broad range of macromolecules. A prominent feature of radicals is that they have extremely high chemical reactivity, which explains not only their normal biological activities, but how they inflict damage on cells.
Oxygen Radicals
There are many types of radicals, but those of most concern in biological systems are derived from oxygen, and known collectively as reactive oxygen species. Oxygen has two unpaired electrons in separate orbitals in its outer shell. This electronic structure makes oxygen especially susceptible to radical formation.
Sequential reduction of molecular oxygen (equivalent to sequential addition of electrons) leads to formation of a group of reactive oxygen species:
- superoxide anion
- peroxide (hydrogen peroxide)
- hydroxyl radical
The structure of these radicals is shown in the figure below, along with the notation used to denote them. Note the difference between hydroxyl radical and hydroxyl ion, which is not a radical.
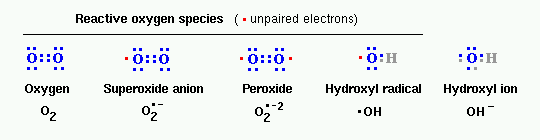
Another radical derived from oxygen is singlet oxygen, designated as 1O2. This is an excited form of oxygen in which one of the electrons jumps to a superior orbital following absorption of energy.
Formation of Reactive Oxygen Species
Oxygen-derived radicals are generated constantly as part of normal aerobic life. They are formed in mitochondria as oxygen is reduced along the electron transport chain. Reactive oxygen species are also formed as necessary intermediates in a variety of enzyme reactions. Examples of situations in which oxygen radicals are overproduced in cells include:
- White blood cells such as neutrophils specialize in producing oxygen radicals, which are used in host defense to kill invading pathogens.
- Cells exposed to abnormal environments such as hypoxia or hyperoxia generate abundant and often damaging reactive oxygen species. A number of drugs have oxidizing effects on cells and lead to production of oxygen radicals.
- Ionizing radiation is well known to generate oxygen radicals within biological systems. Interestingly, the damaging effects of radiation are higher in well oxygenated tissues than in tissues deficient in oxygen.
Biological Effects of Reactive Oxygen
It is best not to think of oxygen radicals as "bad". They are generated in a number of reactions essential to life and, as mentioned above, phagocytic cells generate radicals to kill invading pathogens. There is also a large body evidence indicating that oxygen radicals are involved in intercellular and intracellular signalling. For example, addition of superoxide or hydrogen peroxide to a variety of cultured cells leads to an increased rate of DNA replication and cell proliferation - in other words, these radicals function as mitogens.
Despite their beneficial activities, reactive oxygen species clearly can be toxic to cells. By definition, radicals possess an unpaired electron, which makes them highly reactive and thereby able to damage all macromolecules, including lipids, proteins and nucleic acids.
One of the best known toxic effects of oxygen radicals is damage to cellular membranes (plasma, mitochondrial and endomembrane systems), which is initiated by a process known as lipid peroxidation. A common target for peroxidation is unsaturated fatty acids present in membrane phospholipids. A peroxidation reaction involving a fatty acid is depicted in the figure below.
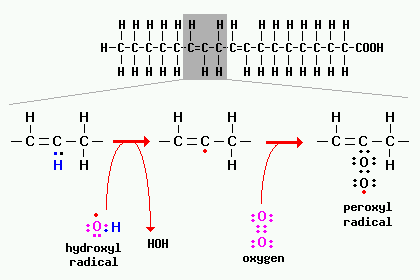
Reactions involving radicals occur in chain reactions. Note in the figure above that a hydrogen is abstracted from the fatty acid by hydroxyl radical, leaving a carbon-centered radical as part of the fatty acid. That radical then reacts with oxygen to yield the peroxy radical, which can then react with other fatty acids or proteins.
Peroxidation of membrane lipids can have numerous effects, including:
- increased membrane rigidity
- decreased activity of membrane-bound enzymes (e.g. sodium pumps)
- altered activity of membrane receptors.
- altered permiability
In addition to effects on phospholipids, radicals can also directly attack membrane proteins and induce lipid-lipid, lipid-protein and protein-protein crosslinking, all of which obviously have effects on membrane function.
Mechanisms for Protection Against Radicals
Life on Earth evolved in the presence of oxygen, and necessarily adapted by evolution of a large battery of antioxidant systems. Some of these antioxidant molecules are present in all lifeforms examined, from bacteria to mammals, indicating their appearance early in the history of life.
Many antioxidants work by transiently becoming radicals themselves. These molecules are usually part of a larger network of cooperating antioxidants that end up regenerating the original antioxidant. For example, vitamin E becomes a radical, but is regenerated through the activity of the antioxidants vitamin C and glutathione.
Enzymatic Antioxidants
Three groups of enzymes play significant roles in protecting cells from oxidant stress:
- Superoxide dismutases (SOD) are enzymes that catalyze the conversion of two superoxides into hydrogen peroxide and oxygen. The benefit here is that hydrogen peroxide is substantially less toxic that superoxide. SOD accelerates this detoxifying reaction roughly 10,000-fold over the non-catalyzed reaction.

SODs are metal-containing enzymes that depend on a bound manganese, copper or zinc for their antioxidant activity. In mammals, the manganese-containing enzyme is most abundant in mitochondria, while the zinc or copper forms predominant in cytoplasm. Interestingly, SODs are inducible enzymes - exposure of bacteria or vertebrate cells to higher concentrations of oxygen results in rapid increases in the concentration of SOD. - Catalase is found in peroxisomes in eucaryotic cells. It degrades hydrogen peroxide to water and oxygen, and hence finishes the detoxification reaction started by SOD.
- Glutathione peroxidase is a group of enzymes, the most abundant of which contain selenium. These enyzmes, like catalase, degrade hydrogen peroxide. They also reduce organic peroxides to alcohols, providing another route for eliminating toxic oxidants.
In addition to these enzymes, glutathione transferase, ceruloplasmin, hemoxygenase and possibly several other enzymes may participate in enzymatic control of oxygen radicals and their products.
Non-enzymatic Antioxidants
Three non-enzymatic antioxidants of particular importance are:
- Vitamin E is the major lipid-soluble antioxidant, and plays a vital role in protecting membranes from oxidative damage. Its primary activity is to trap peroxy radicals in cellular membranes.
- Vitamin C or ascorbic acid is a water-soluble antioxidant that can reduce radicals from a variety of sources. It also appears to participate in recycling vitamin E radicals. Interestingly, vitamin C also functions as a pro-oxidant under certain circumstances.
- Glutathione may well be the most important intracellular defense against damage by reactive oxygen species. It is a tripeptide (glutamyl-cysteinyl-glycine). The cysteine provides an exposed free sulphydryl group (SH) that is very reactive, providing an abundant target for radical attack. Reaction with radicals oxidizes glutathione, but the reduced form is regenerated in a redox cycle involving glutathione reductase and the electron acceptor NADPH.
In addition to these "big three", there are numerous small molecules that function as antioxidants. Examples include bilrubin, uric acid, flavonoids and carotenoids.
Send comments to Richard.Bowen@colostate.edu