VIVO Pathophysiology
Other Topics
Protein Kinase A
Like other protein kinases, protein kinase A (also known as the cyclic AMP-dependent protein kinase or A kinase) is an enzyme that covalently decorates proteins with phosphate groups. The unique characteristic of protein kinase A is that its activity is regulated by fluctuating levels of cyclic AMP within cells (hence its alias as the cyclic AMP-dependent protein kinase). This enzyme thus functions as the end effector for a variety of hormones that work through a cyclic AMP signalling pathway. To put it another way, protein kinase A is ultimately responsible for essentially all of the cellular responses due to the cyclic AMP second messenger system.
Structure
The protein kinase A holoenzyme is a heterotetramer composed of two types of subunits:
- Catalytic subunit: This subunit contains the enzyme's active site. It also contains a domain that binds ATP (the source of phosphate) and a domain that binds the regulatory subunit.
- Regulatory subunit: Two molecules of this subunit bind one another in an anti-parallel orientation to form a homodimer; for type I subunits (see below), this binding is covalent via disulfide bonds. This subunit also has has two domains that bind cyclic AMP, a domain that interacts with a catalytic subunit, and a "auto-inhibitory" domain that serves as a substrate or pseudosubstrate for the catalytic subunit. Regulatory subunits may also have biologic activity distinct from their role in modulating catalytic subunit activity.
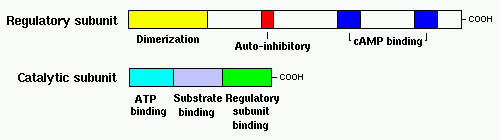
Regulatory subunits exist in two major forms, RI and RII, with each form having two subtypes designated alpha and beta. Each of the four isotypes of the regulatory subunit are encoded by a different gene. In addition, three isotypes of the catalytic subunit have been identified (alpha, beta and gamma). The different isotypes tend to have different distributions within cells and among tissues. Type I enzymes inhabit cytoplasmic, soluble fractions of the cell, whereas type II enzymes tend to associate with cellular membranes.
Regulation of Activity
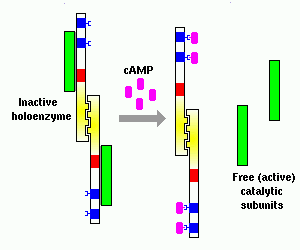
Intracellular concentration of cyclic AMP provides the most fundamental control over activity of protein kinase A:
|
 |
Protein kinase A often acts at very discrete domains within cells. Such spatial targeting results from interaction of type I regulatory subunits with proteins called A kinase anchoring proteins (AKAPs). A large number of distinct AKAPs have been identified and shown to colocalize protein kinase A to some of its specific substrates, including ion channels, cytoskeletal elements and centrosomes. In some cases, AKAPs also bind other molecules involved in the cyclic AMP signalling pathway, including different phosphodiesterases, which destroy cyclic AMP. By sequestering both protein kinase A and an enzyme that ultimately turns it off into specific locations, phosphorylation events can be very carefully controlled.
The activity of protein kinase A is also modulated by a group of proteins called protein kinase inhibitors. These molecules often act as pseudosubstrates for the catalytic subunit, "distracting" it from legitimate phosphorylation targets.
Phosphorylation Targets
The catalytic subunits of protein kinase A phosphorylate proteins at serine and threonine residues; the usual target sequence is [Arg-Arg-X-Ser/Thr-X], where X is a hydrophobic amino acid. Protein kinase A phosphorylates substrates in both the cytoplasm and nucleus.
Protein kinase A phosphorylates and thereby changes the activity of a number of important molecules. Included in its target list are:
- Enzymes: Phosphorylation is widely used as a molecular switching mechanism to activate or inactivate enzyme activity. In many cases, the enzyme being phosphorylated is itself a kinase. The classical example is that protein kinase A phosphorylates the enzyme phosphorylase kinase, which, in turn, phosphorylates glycogen phorphorylase, which leads to breakdown of glycogen in liver and muscle.
- Ion channels: Certain calcium channels in cardiac muscle cells are activated by protein kinase A, ultimately leading to muscle contraction. Another medically important example is that protein kinase A phosphorylates and thus activates a chloride channel important in secretion of water in the small intestine.
- Chromosomal proteins: Histone H1 was the first target identified for protein kinase A.
- Transcription factors: CREB's (cyclic AMP response element binding proteins) are transcription factors that, when phosphorylated by protein kinase A, become competent to bind promoter regions of responsive genes and stimulate transcription.
This short list gives but a taste of the importance of protein kinase A in such essential processes as energy metabolism, muscle contraction, membrane transport and gene expression.
References and Reviews
- Bauman AL, Scott JD: Kinase- and phosphatase-anchoring proteins: harnessing the dynamic duo. Nature Cell Biol 4:E203-206, 2002.
- Chin K, Yang W, Ravatn R, etc. Reinventing the Wheel of cyclic AMP: Novel Mechanisms of cAMP Signaling. Ann New York Acad Sci 968:49-64, 2002.
- Johnson DA, Akamine P, Radzio-Andzelm E, Madhusudan, Taylor SS: Dynamics of cAMP-dependent protein kinase. Chem Rev 101:2243-2270, 2001.
- Robinson-White A, Stratakis CA: Protein Kinase A Signaling: "Cross-Talk" with Other Pathways in Endocrine Cells. Ann New York Acad Sci 968:256-270, 2002.
- Shabb JB. Physiological substrates of cAMP-dependent protein kinase. Chem Rev 101:2381-2411, 2001.
Send comments to Richard.Bowen@colostate.edu