VIVO Pathophysiology
Mechanism of Action: Hormones with Cell Surface Receptors
Protein and peptide hormones, catecholamines like epinephrine, and eicosanoids such as prostaglandins find their receptors decorating the plasma membrane of target cells.
Binding of hormone to receptor initiates a series of events which leads to generation of so-called second messengers within the cell (the hormone is the first messenger). The second messengers then trigger a series of molecular interactions that alter the physiologic state of the cell. Another term used to describe this entire process is signal transduction.
Structure of Cell Surface Receptors
Cell surface receptors are integral membrane proteins and, as such, have regions that contribute to three basic domains:
- Extracellular domains: Some of the residues exposed to the outside of the cell interact with and bind the hormone - another term for these regions is the ligand-binding domain.
- Transmembrane domains: Hydrophobic stretches of amino acids are "comfortable" in the lipid bilayer and serve to anchor the receptor in the membrane.
- Cytoplasmic or intracellular domains: Tails or loops of the receptor that are within the cytoplasm react to hormone binding by interacting in some way with other molecules, leading to generation of second messengers. Cytoplasmic residues of the receptor are thus the effector region of the molecule.
Several distinctive variations in receptor structure have been identified. As depicted below, some receptors are simple, single-pass proteins; many growth factor receptors take this form. Others, such as the receptor for insulin, have more than one subunit. Another class, which includes the beta-adrenergic receptor, is threaded through the membrane seven times.
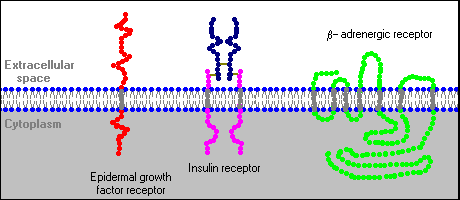
Receptor molecules are neither isolated by themselves nor fixed in one location of the plasma membrane. In some cases, other integral membrane proteins interact with the receptor to modulate its activity. Some types of receptors cluster together in the membrane after binding hormone. Finally, as elaborated below, interaction of the hormone-bound receptor with other membrane or cytoplasmic proteins is the key to generation of second messengers and transduction of the hormonal signal.
Second Messenger Systems
Consider what would happen if, late at night, you noticed a building on fire. Hopefully, you would dial 911 or a similar emergency number. You would inform the dispatcher of the fire, and the dispatcher would, in turn, contact and "activate" a number of firemen. The firefighters would then rapidly go to work pouring water on the fire, setting up roadblocks and the like. They would also probably activate other "players", such as police and fire investigators that would come in later to try and determine the cause of the fire. Importantly, once the fire is out (or the building totally destroyed), the firemen go back to the station and to sleep.
The community response to a fire is, at least in some ways, analogous to a second messenger system involved in a hormone's action. In the scenario described, you are the "first messenger", the dispatcher is "receptor", the firefighters are "second messengers".
Currently, four second messenger systems are recognized in cells, as summarized in the table below. Note that not only do multiple hormones utilize the same second messenger system, but a single hormone can utilize more than one system. Understanding how cells integrate signals from several hormones into a coherent biological response remains a challenge.
| Second Messenger | Examples of Hormones Which Utilize This System |
|---|---|
| Cyclic AMP | Epinephrine and norepinephrine, glucagon, luteinizing hormone, follicle stimulating hormone, thyroid-stimulating hormone, calcitonin, parathyroid hormone, antidiuretic hormone |
| Protein kinase activity | Insulin, growth hormone, prolactin, oxytocin, erythropoietin, several growth factors |
| Calcium and/or phosphoinositides | Epinephrine and norepinephrine, angiotensin II, antidiuretic hormone, gonadotropin-releasing hormone, thyroid-releasing hormone. |
| Cyclic GMP | Atrial naturetic hormone, nitric oxide |
In all cases, the seemingly small signal generated by hormone binding its receptor is amplified within the cell into a cascade of actions that changes the cell's physiologic state. Presented below are two examples of second messenger systems commonly used by hormones. The examples used are of glucagon and insulin, both of which ultimately work through a molecular switch involving protein phosphorylation. Be aware that in both cases, a very complex system is being simplified considerably.
Cyclic AMP Second Messenger Systems
Cyclic adenosine monophosphate (cAMP) is a nucleotide generated from ATP through the action of the enzyme adenylate cyclase. The intracellular concentration of cAMP is increased or decreased by a variety of hormones and such fluctuations affect a variety of cellular processes. One prominent and important effect of elevated concentrations of cAMP is activation of a cAMP-dependent protein kinase called protein kinase A.
Protein kinase A is nominally in an catalytically-inactive state, but becomes active when it binds cAMP. Upon activation, protein kinase A phosphorylates a number of other proteins, many of which are themselves enzymes that are either activated or suppressed by being phosphorylated. Such changes in enzymatic activity within the cell clearly alter its state.
Now, let's put this information together to understand the mechanism of action of a hormone like glucagon:
- Glucagon binds its receptor in the plasma membrane of target cells (e.g. hepatocytes).
- Bound receptor interacts with and, through a set of G proteins, turns on adenylate cyclase, which is also an integral membrane protein.
- Activated adenylate cyclase begins to convert ATP to cyclic AMP, resulting in an elevated intracellular concentration of cAMP.
- High levels of cAMP in the cytosol make it probable that protein kinase A will be bound by cAMP and therefore catalytically active.
- Active protein kinase A "runs around the cell" adding phosphates to other enzymes, thereby changing their conformation and modulating their catalytic activity - - - abracadabra, the cell has been changed!
- Levels of cAMP decrease due to destruction by cAMP-phosphodiesterase and the inactivation of adenylate cyclase.
In the above example, the hormone's action was to modify the activity of pre-existing components in the cell. Elevations in cAMP also have important effects on transcription of certain genes.
Tyrosine Kinase Second Messenger Systems
The receptors for several protein hormones are themselves protein kinases which are switched on by binding of hormone. The kinase activity associated with such receptors results in phosphorylation of tyrosine residues on other proteins. Insulin is an example of a hormone whose receptor is a tyrosine kinase.
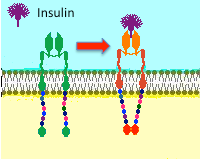
The hormone binds to domains exposed on the cell's surface, resulting in a conformational change that activates kinase domains located in the cytoplasmic regions of the receptor. In many cases, the receptor phosphorylates itself as part of the kinase activation process. The activated receptor phosphorylates a variety of intracellular targets, many of which are enzymes that become activated or are inactivated upon phosphorylation.
|
The cartoon to the right is meant to depict a tyrosine kinase receptor like that used by insulin. Following binding of hormone, the receptor undergoes a conformational change, phosphorylates itself, then phosphorylates a variety of intracellular targets. |
|
As was seen with cAMP second messenger systems, activation of receptor tyrosine kinases leads to rapid modulation in a number of target proteins within the cell. Interestingly, some of the targets of receptor kinases are protein phosphatases which, upon activation by receptor tyrosine kinase, become competent to remove phosphates from other proteins and alter their activity. Again, a seemingly small change due to hormone binding is amplified into a multitude of effects within the cell.
In some cases, binding of hormone to a surface receptor induces a tyrosine kinase cascade even through the receptor is not itself a tyrosine kinase. The growth hormone receptor is one example of such a system - the interaction of growth hormone with its receptor leads to activation of cytoplasmic tyrosine kinases, with results conceptually similar to that seen with receptor kinases.
Fate of the Hormone-Receptor Complex
Normal cell function depends upon second messenger cascades being transient events. Indeed, a number of cancers are associated with receptors that continually stimulate second messenger systems. One important part of negative regulation on hormone action is that cell surface receptors are internalized. In many cases, internalization is stimuated by hormone binding.
Internalization occurs by endocytosis through structures called coated pits. The resulting endosomes (sometimes called "receptosomes") may fuse with lysosomes, leading to destruction of the receptor and hormone. In other cases, it appears that the hormone dissociates and the receptor is recycled by fusion of the endosome back into the plasma membrane.
Send comments to Richard.Bowen@colostate.edu

