VIVO Pathophysiology
Small Intestinal Brush Border Enzymes
The final step in digestion of dietary carbohydrates and proteins occurs on the face of small intestinal enterocytes, in the immediate vicinity of the transporters which will ferry the resulting sugars and amino acids into the epithelial cells. The enzymes responsible for this terminal stage of digestion are not free in the intestinal lumen, but rather, tethered as integral membrane proteins in the plasma membrane of the enterocyte. The apical plasma membrane housing these enzymes is composed of numerous microvilli which extend from the cell and constitute the "brush border". Hence, the enzymes embedded in those microvilli are referred to as brush border enzymes.
The density and distribution of brush border enzymes differs among different segments of the small intestine and often varies depending on the age of the animal. Additionally, in some cases the concentration of such enzymes can be modulated by diet; for example, the amount of sucrase-isomaltase enzyme increases in animals fed a high-carbohydrate diet.
Maltase-Glucoamylase and Sucrase-Isomaltase
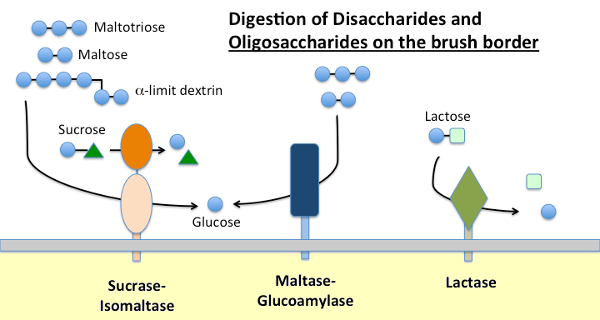
Dietary starch is digested by α-amylase present in pancreatic secretions and, in many species, saliva. Amylase hydrolyzes internal α-1,4 glycosidic bonds in starch to generate maltose, short oligosaccharides (e.g. maltotriose) and so-called limit dextrins, which are branch points in amylopectin. None of these molecules can be absorbed across the small intestinal epithelium and require further hydrolysis, ultimately into glucose.
Maltase-glucoamylase and sucrase-isomaltase are closely related enzymes embedded in the brush border membrane that execute the terminal stages of digestion using substrates provided by the action of amylase:
- Maltase-glycoamylase has two active sites. The maltase site hydrolyzes terminal α-1,4-linked D-glucose residues from maltose or maltotriose, generating α-D-glucose. The glucoamylase site also has that α-1,4 hydrolase activity but also hydrolyses α-1,6 glycosidic linkages when they are adjacent to an α-1,4 linkage, liberating β-D-glucose.
- Sucrase-isomaltase also has two active sites with different specificities. The sucrase site catalyzes hydrolysis of sucrase into fructose and glucose, and also is capable of hydrolyzing maltose. The isomaltase site is important as the major mechanism for digesting the α-1,6 linkages of the limit dextrins.
Lactase
Lactase, as its name implies is a disaccharidase that hydrolyses lactose into galactose and glucose; formally, it is a β-glycosidase. Lactose is the major carbohydrate in milk and lactase is abundantly present in the small intestinal brush border of young mammals. In most species, including humans, expression of lactase plummets shortly after weaning. In a majority of humans, this non-persistence of lactase activity in adulthood is associated with "lactose intolerance". The difference between lactase persistence and non-persistence is genetically determined.
Peptidases
There are a large number of brush border peptidases, which collectively can hydrolyze the diverse amino acid sequence diversity present in dietary proteins. Major classes of peptidases include:
- Exopeptidases that hydrolyze terminal amino acids from peptides. Some of these enzymes have activity against C-terminal residues and others work on N-terminal amino acids. Their activity yields free amino acids or dipeptides. As an example of enzyme specificity and diversity, aminopeptidase P hydrolyzes N-terminal amino acid from di-, tri- and oligopeptides but only if they are linked to proline, whereas aminopeptidase A catalyzes the hydrolysis of terminal acidic amino acids such as glutamate and aspartate.
- Endopeptidases cleave peptide chains internally, yielding smaller peptides of varying chain length.
- A single dipeptidase also exists as a brush border enzyme.
Enteropeptidase, also known as enterokinase, is another brush border enzyme that has the important activity of catalyzing the activiation of trypsinogen into trypsin, one of the major proteases from the pancreas. Enteropeptidase is present most abundantly in the duodenum.
Lipases
A number of lipases are present in the brush border of the small intestine. These include phospholipase B1, neutral ceramidase and alkaline sphingomyelinase.
References and Reviews
- Hooton D, Lentle R, Monro J, Wickham M, Simpson R. The secretion and action of brush border enzymes in the mammalian small intestine. Rev Physiol Biochem Pharmacol 2015; 168:59–118.
Send comments to Richard.Bowen@colostate.edu
